Published: Darrell O. Ricke. Vaccine-associated Kawasaki disease in children. Microbes & Immunity 025200044. https://doi.org/10.36922/MI025200044
I have recently published and posted on an unusual pattern of increased risk of epilepsy, bradycardia (slow heart rate), and cardiac arrest in children associated with (1) specific vaccines, (2) specific vaccine combinations, and (3) child age. I was surprised to see that this pattern also extended to Kawasaki Disease in children - the focus of this post.
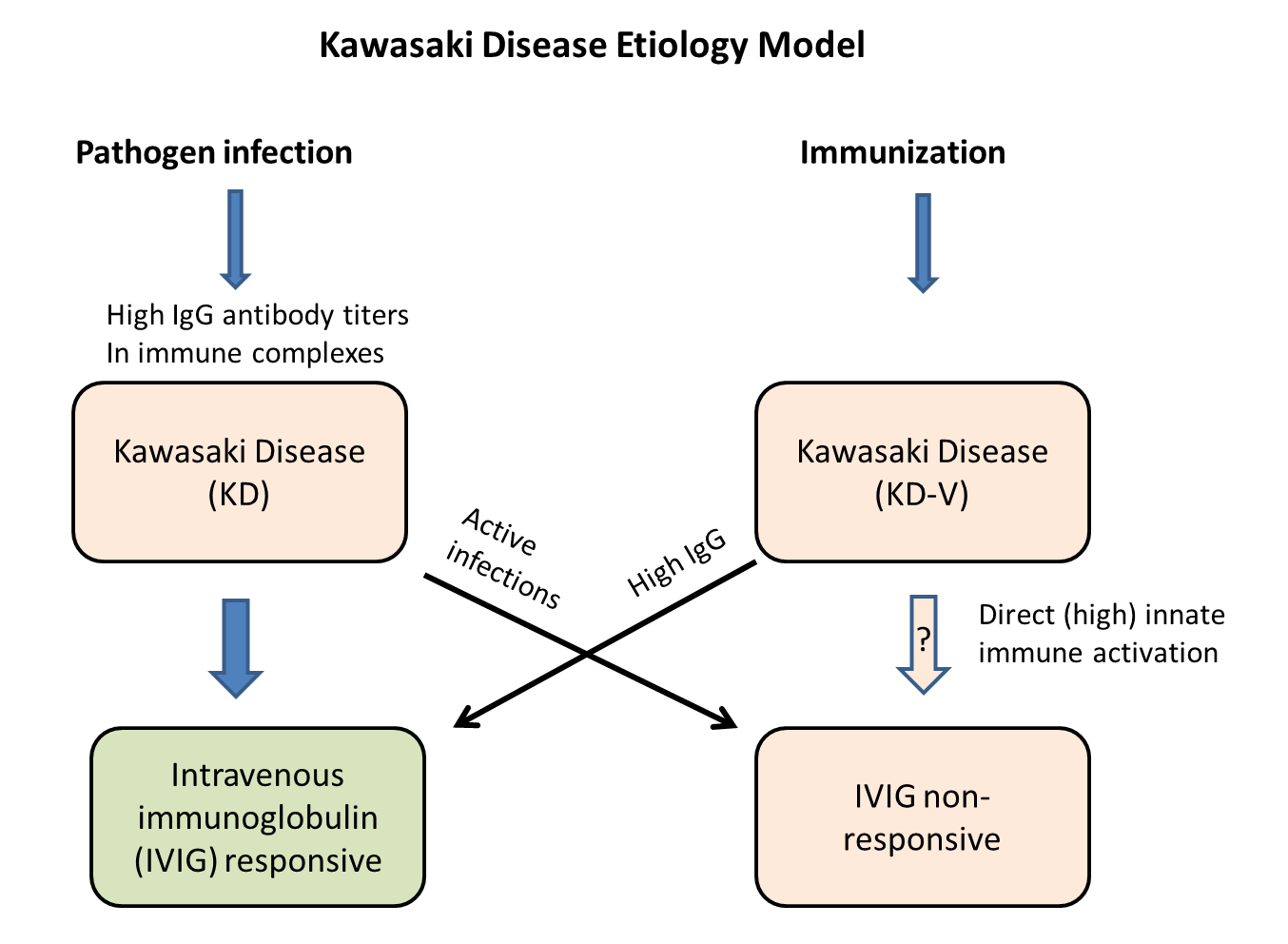
Kawasaki disease is the leading cause of acquired heart disease in children in developed countries. The cause and mechanisms of Kawasaki disease are unknown. I have previously published and proposed a model for this disease - see:
Ricke, D.O.; Smith, N. VAERS Vasculitis Adverse Events Retrospective Study: Etiology Model of Immune Complexes Activating Fc Receptors in Kawasaki Disease and Multisystem Inflammatory Syndromes. Life 2024, 14, 353. https://doi.org/10.3390/life14030353
Two of the main treatments for Kawasaki disease are (1) bulk intravenous immunoglobulin (IVIG) and (2) high dose aspirin. The medical community doesn’t understand the mechanisms for either of these treatments (I’ve proposed models in the above article). For pathogen associated Kawasaki disease, the IVIG works by competing against immune complexes (antibodies bound to pathogens or vaccine proteins) binding to Fc receptors on immune cells (mast cells) and platelets (activation releases histamine, serotonin, etc.). Aspirin is a known mast cell stabilizer. Aspirin is normally not give to children due to the risk of Reye’s syndrome.
This latest article provides new ideas that possibly explains two aspects of Kawasaki disease:
Excess of Kawasaki disease in children
Why some Kawasaki patients are not response to IVIG treatments.
Graphical abstract
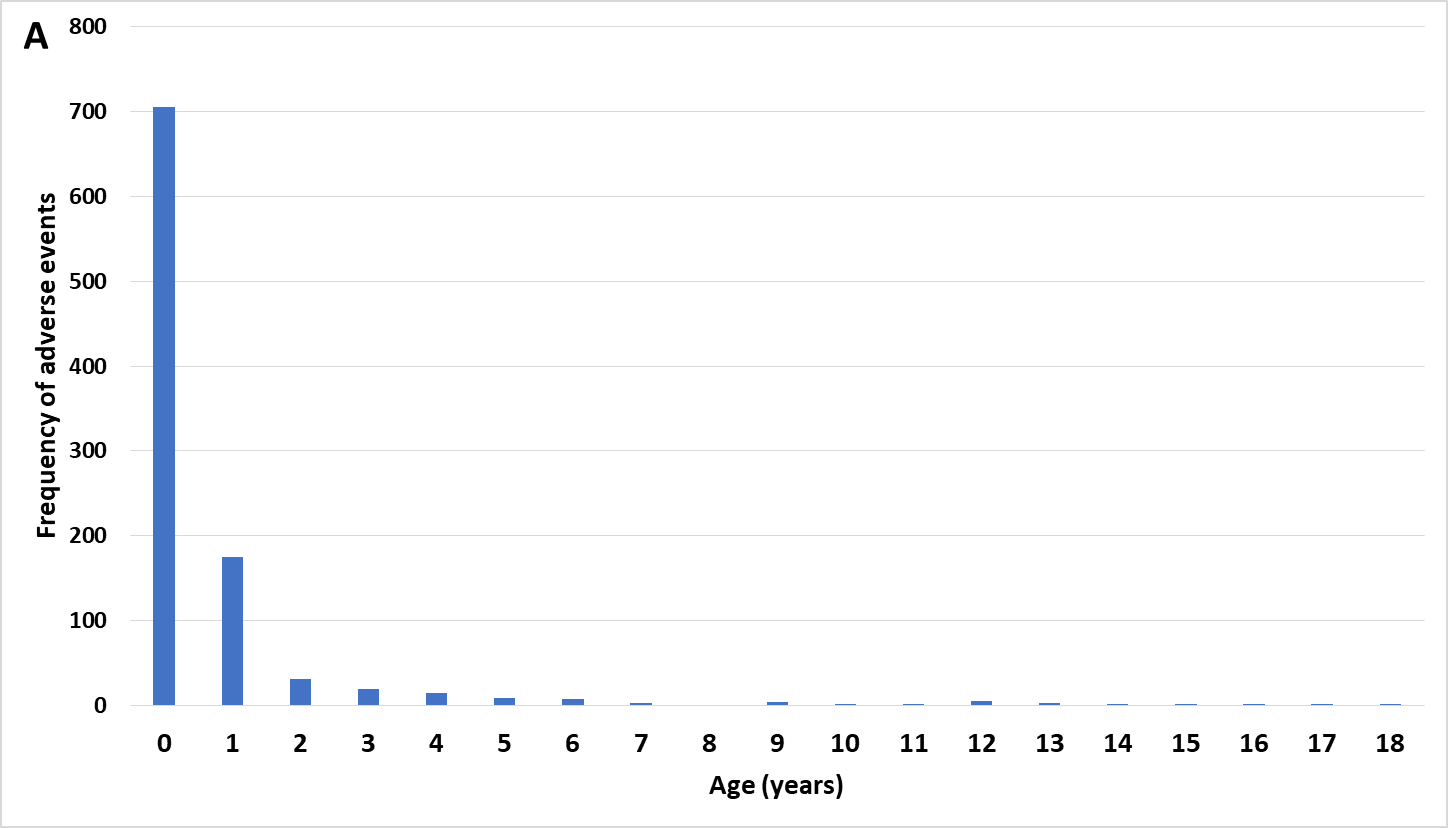
Figure 1 (A) Kawasaki’s Disease adverse events by age and (B) Kawasaki’s Disease day of onset post immunization
Note that the majority of the children are ages 0 (infants) and 1! This is the pattern that matched this data to the infant safety series pattern. My hypothesis is that when this is occurring, the risk of the vaccine or combined vaccines dosage(s) being too high is consistent with this pattern - in other words, the immune system appears to be getting over activated and Kawasaki disease is the outcome for these children.
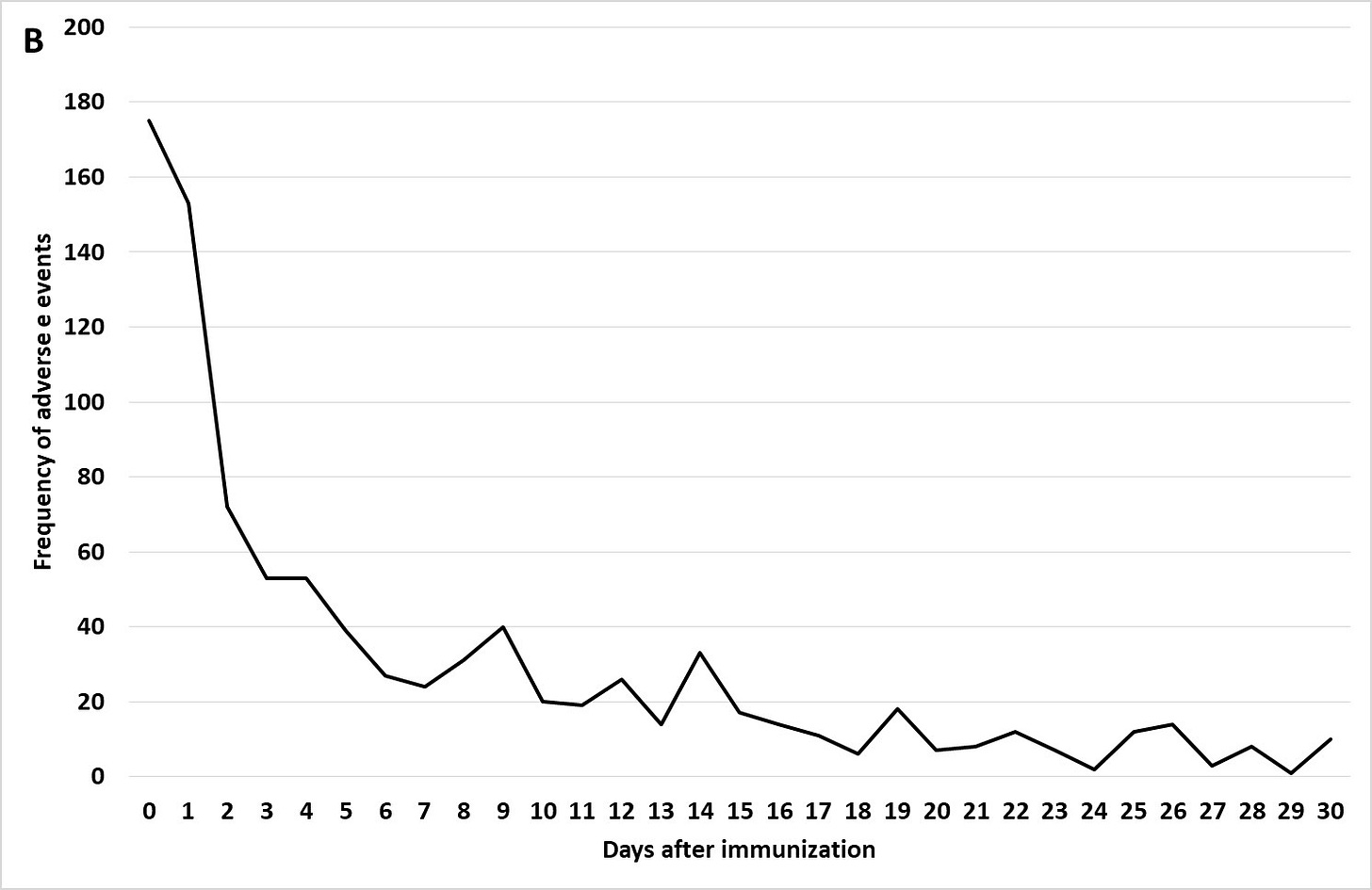
Figure 1 (B) Kawasaki’s Disease day of onset post immunization
Note the immediate onset of Kawasaki disease with the first day or two for most children after immunization! Reporting bias can apply to the onset reports to VAERS, but this is rapid!
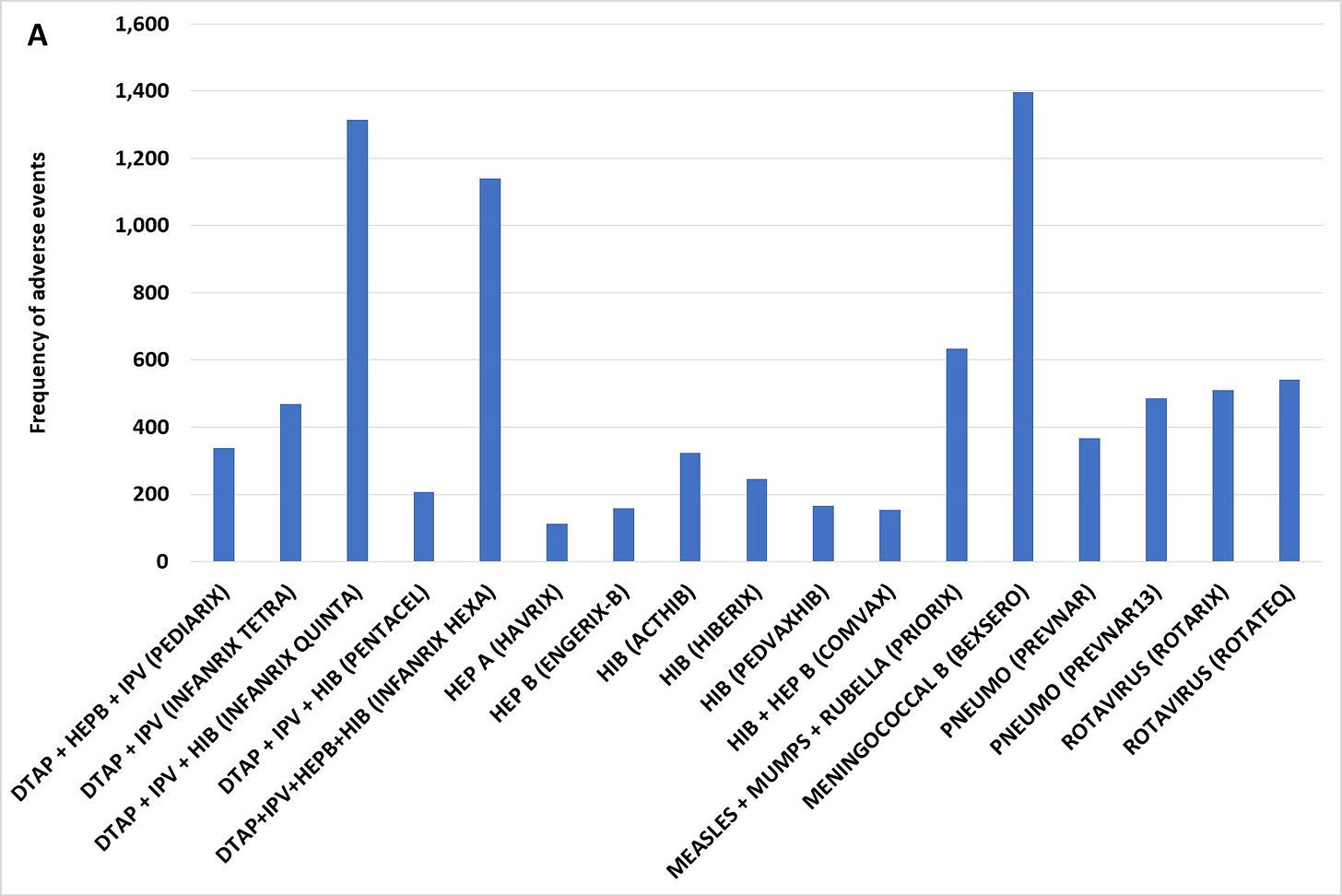
Figure 2 (A) Kawasaki’s Disease adverse events post immunization by vaccine for children age 10 and younger scaled to 100,000 total VAERS reports per vaccine
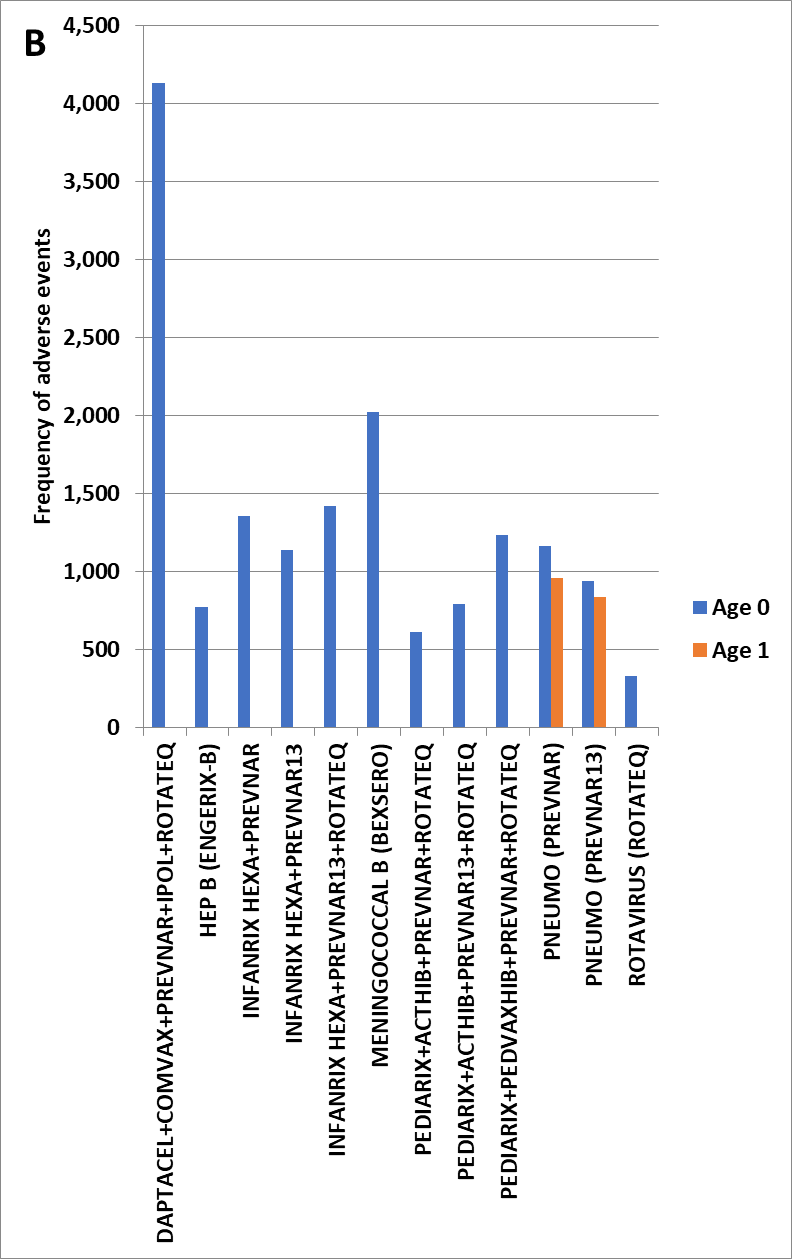
Figure 2 (B) Kawasaki’s Disease adverse events by child age (0 to 1) and vaccine combination scaled to 100,000 total VAERS reports per vaccine or combination
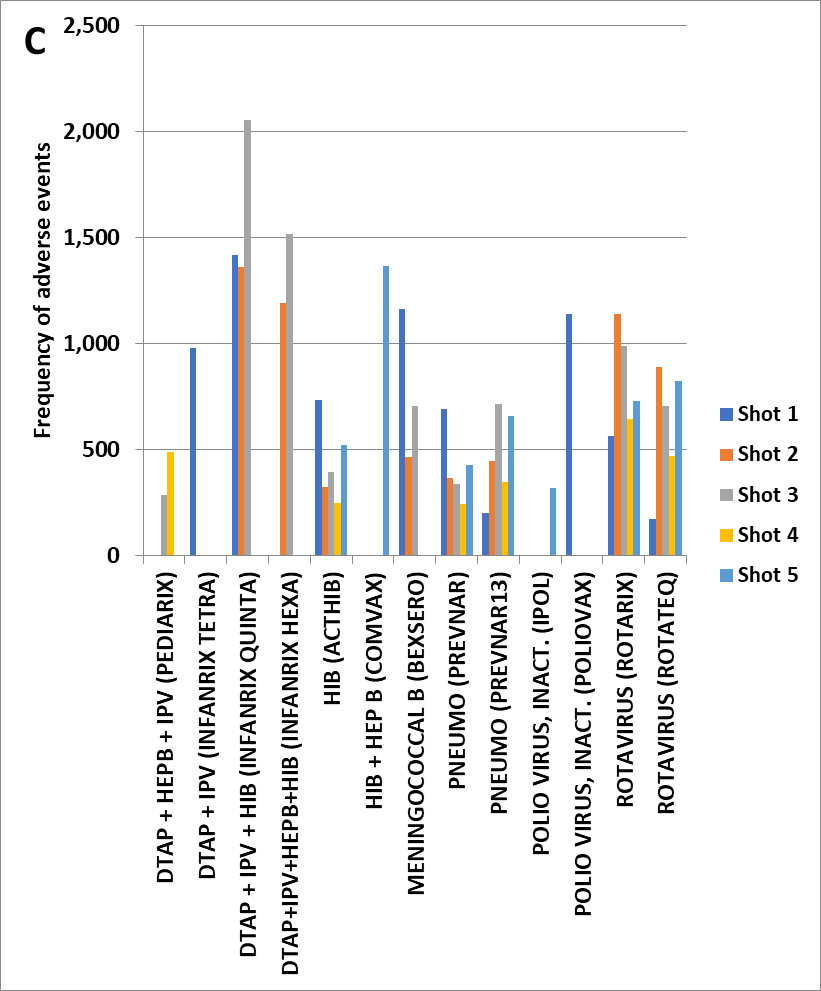
Figure 2 (C) Kawasaki’s Disease by vaccine dose to age 10 scaled to 100,000 total VAERS reports per vaccine
Hypothesis 1: Kawasaki’s Disease is caused by release of elevated histamine levels and possibly serotonin released from activated mast cells and likely platelets 2. One mechanism for activation is immune complexes binding to the Fc receptors on mast cells and platelets.
Hypothesis 2: Immunization can trigger Kawasaki’s Disease by either (1) direct activation of mast cells from immune responses or by (2) immune complexes of antibodies binding to vaccine antigens.
Hypothesis 3: Kawasaki’s Disease patients that do not respond to IVIG treatment may be due to (1) ongoing KD-causing pathogen infection (e.g., ongoing high KD pathogen immune complexes concentrations) or (2) KD caused by direct activation of mast cells and possibly platelets from immune responses.
Hypothesis 4: Over activation of immune responses is causing Kawasaki disease post immunization (KD-V) in children. With lack of dose adjustment based on child’s body size (age), KD AEFIs can result (Figure 1A) for specific vaccines, live attenuated viral vaccines with infectious units not adjusted for child body size, and multiple specific concomitant vaccine combinations (including increased concentrations of adjuvant components – e.g., aluminum).
The onset data (Figure 1B) and multiple dose results (Figure 2C) support models of activation of mast cells and likely platelets by (1) direct innate immune responses supporting Hypothesis 2 and (2) immune complex activation of Fc receptors supporting Hypothesis 1. Treatments of KD patients by IVIG in the context of direct innate immune response activations is predicted to appear as IVIG non-responders. And, relevant to Hypothesis 1, IVIG treatment should act as competitive antibodies for Fc receptor binding that reduces activation for the alternative activation pathway; but, for ongoing infections (including live vaccines), IVIG treatment may appear as non-responsive because of ongoing creation of immune complexes. If the proposed KD etiology models are correct, then additional adjunctive treatments including mast cell stabilizers, anti-histamines, and possibly serotonin antagonists may reduce KD symptoms; institutional review board (IRB) approved targeted clinical studies (e.g., case series) are suggested (perhaps focusing on IVIG non-responders).
Key causes to investigate/consider:
Vaccine doses and infant body size/weight - this is a corner stone for medicine
Total aluminum concentration for multiple vaccines administered at one time - see articles and Substack posts by Dr. Christopher Exley
Combining live attenuated virus vaccines with other (aluminum adjuvanted) vaccines
Possible manufacturing contamination (e.g., endotoxins - see Substack posts by Dr. Geoff Pain) in vaccines identified in this article
This is a free substack.